3d Bohr Model Of Iron
Wednesday, May 3, 2023
Edit
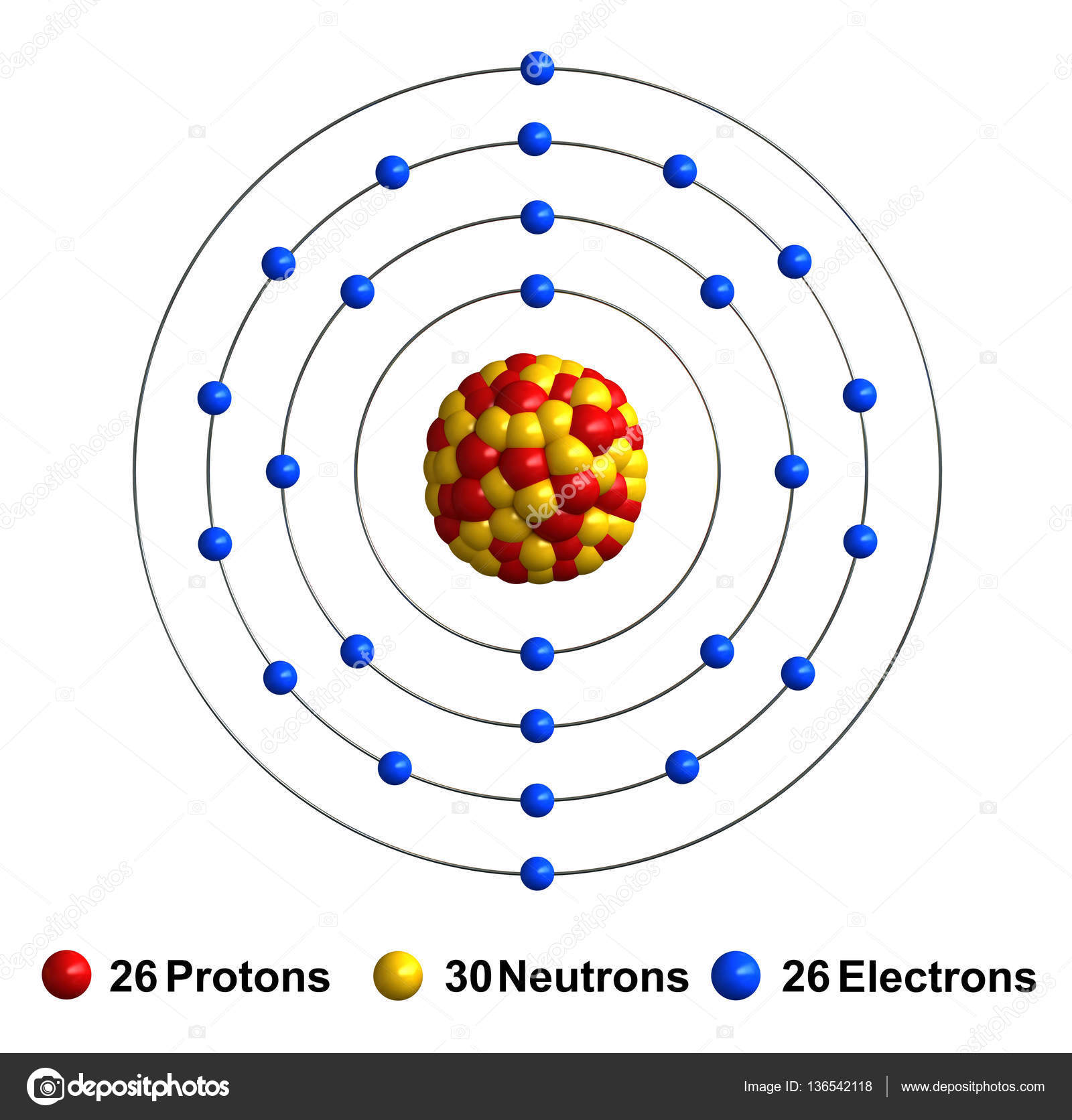
3D Bohr Model of Iron
What is a 3D Bohr Model?
The 3D Bohr Model is a way of representing the structure of an atom using a 3-dimensional model. It is based on the Bohr Model of the atom, which was developed by Danish physicist Niels Bohr in 1913. In this model, the atom is represented as a small, positively charged nucleus surrounded by orbiting electrons. The nucleus is made up of protons and neutrons, while the electrons are arranged in shells or orbitals around the nucleus. The 3D Bohr Model of an atom shows how the electrons are arranged around the nucleus in three dimensions.
What is Iron?
Iron is a chemical element with the symbol Fe. It is a transition metal and is one of the most common elements in the world. Iron is a very strong, malleable metal, and it is the fourth most abundant element in the Earth's crust. Iron is used to make steel, which is used in construction, tools, and other products. Iron is also a vital part of hemoglobin, the protein that carries oxygen in our blood.
The 3D Bohr Model of Iron
The 3D Bohr Model of Iron is a representation of the atom's structure. The nucleus of an iron atom contains 26 protons and 30 neutrons, giving it an atomic number of 26 and an atomic mass of 56. In the 3D Bohr Model of Iron, the electrons are arranged in shells or orbitals around the nucleus. The first shell contains two electrons, the second shell contains eight electrons, and the third shell contains 14 electrons.
The Valence Shell of Iron
The valence shell of Iron is the outermost shell, which contains eight electrons. These electrons are the most reactive and most likely to interact with other atoms. In the 3D Bohr Model of Iron, the valence shell is represented as an octahedron, with four of the electrons located in each of the four corners. The valence shell of Iron is responsible for many of the properties of the element, such as its strength and its ability to form bonds with other atoms.
The Bonding Properties of Iron
The 3D Bohr Model of Iron is useful for understanding the bonding properties of the element. Iron can form bonds with other elements in a variety of ways, including covalent bonding and ionic bonding. In covalent bonding, two atoms share electrons in order to form a chemical bond. In ionic bonding, one atom transfers electrons to another atom in order to form a bond. Iron can form both covalent and ionic bonds, depending on the other element it is bonding with.
Uses of Iron
Iron is one of the most important elements on the planet. It is used in a wide variety of applications, from the construction of buildings and bridges to the production of tools and machines. Iron is also an essential component of hemoglobin, the protein that carries oxygen in our blood. Iron is also used to make steel, which is used in everything from cars to ships.
Conclusion
The 3D Bohr Model of Iron is a useful tool for understanding the structure of the element and its bonding properties. The 3D Bohr Model of Iron shows how the electrons are arranged in shells or orbitals around the nucleus and how the valence shell is responsible for many of the properties of the element. Iron is an important and versatile element, used in a variety of applications from the construction of buildings to the production of tools and machines.