3d Bohr Model Of Oxygen
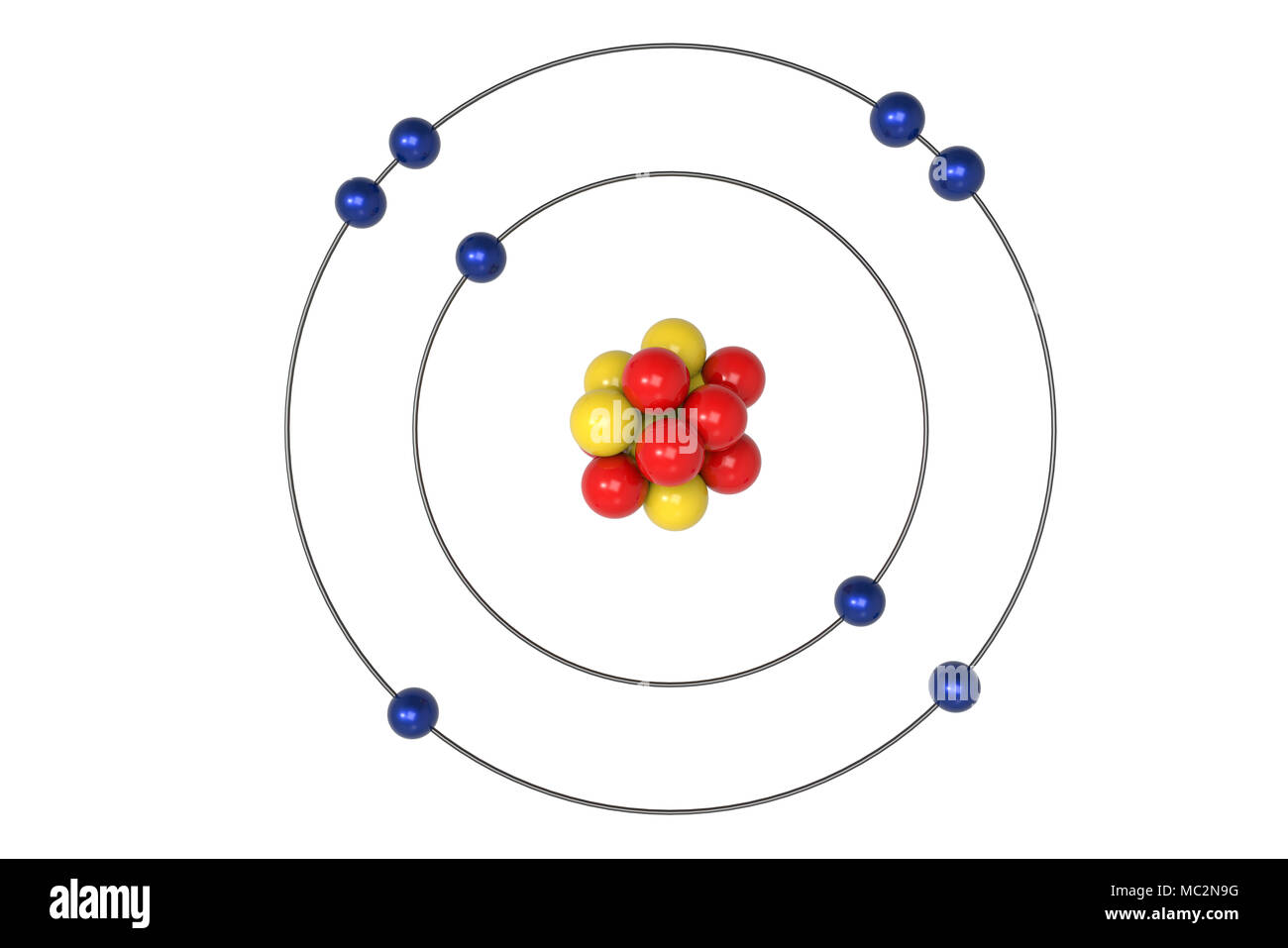
The Basics of the 3D Bohr Model of Oxygen
One of the most interesting and important atoms to study in chemistry is oxygen. It is found in many different forms, from oxygen gas to oxygen molecules in the form of water. Oxygen is also essential for all forms of life, and it takes on several different shapes and forms as it interacts with other atoms and molecules. One of the most interesting ways to view an oxygen atom is through the 3D Bohr Model.
What is the 3D Bohr Model?
The 3D Bohr Model is a way of visualizing the structure of an atom in three dimensions. It was developed by the Danish physicist Niels Bohr in 1913. This model helps to explain the behavior of electrons in an atom, and how they interact with the nucleus. In the 3D Bohr Model, the nucleus is represented by a sphere, and the electrons orbit around it in different shells. Each shell is associated with a certain amount of energy, and the electrons move between different shells depending on the amount of energy they have.
The Structure of the 3D Bohr Model of Oxygen
The 3D Bohr Model of Oxygen has a nucleus made up of eight protons and eight neutrons. This is surrounded by two shells of electrons. The inner shell contains two electrons, and the outer shell contains six electrons. The electrons in the outer shell can move around in different orbits, depending on the amount of energy they have. The electrons in the inner shell are more tightly bound to the nucleus and do not move around as much.
How the 3D Bohr Model of Oxygen is Used
The 3D Bohr Model of Oxygen is a helpful way of understanding how oxygen atoms interact with other atoms and molecules. It helps to explain why oxygen can form bonds with other atoms, and how these bonds affect the properties of molecules. For example, the 3D Bohr Model can help explain why water molecules are held together by hydrogen bonds, and why oxygen gas is so reactive. It also helps to explain why some elements, such as oxygen, are so common in nature.
The Benefits of the 3D Bohr Model of Oxygen
The 3D Bohr Model of Oxygen is a useful tool for understanding the structure of oxygen atoms and how they interact with other atoms. It can help to explain why some elements, such as oxygen, are so common in nature, and how they form bonds with other atoms. It can also help to explain why oxygen gas is so reactive, and why water molecules are held together by hydrogen bonds. The 3D Bohr Model is a helpful way of visualizing the structure of an atom, and it can help to explain many chemical reactions.
Conclusion
The 3D Bohr Model of Oxygen is a helpful way of understanding the structure of oxygen atoms, and how they interact with other atoms. It can help to explain why some elements, such as oxygen, are so common in nature, and why oxygen gas is so reactive. It can also help to explain why water molecules are held together by hydrogen bonds. The 3D Bohr Model is a useful tool for visualizing the structure of an atom, and it can help to explain many chemical reactions.